Early detection saves lives1
Screening matters for your employees
of people diagnosed with CRC in early stages survive2,3
of people diagnosed with CRC in late stages survive2,3
Screening is key to reducing colorectal cancer (CRC) mortality
CRC is the 2nd leading cause of cancer deaths among men and women in the US—yet more than half of eligible workers (age 45-64) are not up to date with CRC screening.2,4
You can remove barriers to screening
Current CRC screening options exist, but they can be time-consuming and uncomfortable, which may be why your employees remain unscreened. Increase CRC screening compliance with an accurate blood-based approach. 1,2,7-10
Shield™, an easy-to-complete blood test12
- Requires no preparation
- No extra time away from work
- Detects CRC with high accuracy


Make a difference and take control
You can now offer eligible employees blood-based screening for CRC, helping to increase screening compliance.
With Shield, a convenient and proven blood test, you can help employees detect CRC early—when it's most treatable.1,10,11
Offer Shield as a benefitProtect your employees and your business
Protect your employees and your business
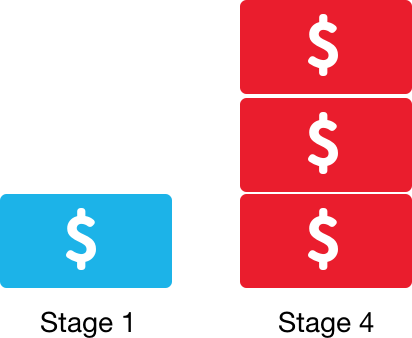
Late-stage CRC treatment is more expensive than early-stage treatment.13
Stage 4 CRC treatment costs 3x as much as CRC Stage 1. CRC screening and early cancer detection helps with productivity and cost management. Screening saves lives, and education is the best way to encourage your employees to get screened.13
Let's get screened
Guardant Health partners with leading employers like you to help drive CRC screening compliance. Shield can be easily integrated or adopted to your current education and awareness programs, worksite health center, and on-site screening programs.
Learn how Shield fits into your organization
Please fill out the information below or email us directly and we will be in touch!
- The assay is intended to be complementary to and not a replacement for current recommended colorectal cancer screening methods
- Patients with an “abnormal signal detected” Shield result should be referred for colonoscopic evaluation
- A “normal signal detected” Shield result does not preclude the presence of colorectal neoplasia, and patients should continue participating in guideline-recommended screening programs
- Shield was developed, and its performance characteristics determined, by the Guardant Health Clinical Laboratory in Redwood City, CA, USA, which is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) as qualified to perform high complexity clinical testing. This test has not been cleared or approved by the US FDA
