You're here to keep your employees healthy.
Shield™—Redefining Colorectal Cancer Screening
Learn more52% of the screening-eligible working-age population is not adherent with guideline recommended Colorectal Cancer (CRC) screening.1 You can help change this.
Shield from Guardant Health is a CRC screening that consists of a simple blood draw. We offer the test either through your onsite clinic or onsite mobile screening events so employees don't have to do anything except show up. No preparation time. No sedation. Less time away from work. Simply done.
Learn more about our easy-to-implement screening options
CRC is the second-leading cause of cancer deaths in the United States2—but it doesn't need to be.
Did you know:
-
1 in 3 adults aged 50-75 are not up-to-date with recommended CRC screening.3
-
More than 75% of people who died from CRC were not up-to-date with screening.4
-
60% of CRC deaths could be prevented with screening.5
5-YEAR SURVIVAL RATE
Based on stage of Diagnosis5

Now we can do better
As an employer, you have an opportunity to help contribute to the health of your employees by empowering them to get screened and take control of their health.

Shield™ is a blood-based test that requires no preparation time and less time away from work
-
Simple blood draw
-
Sample sent to Guardant Health Laboratory
-
Receive results within two weeks
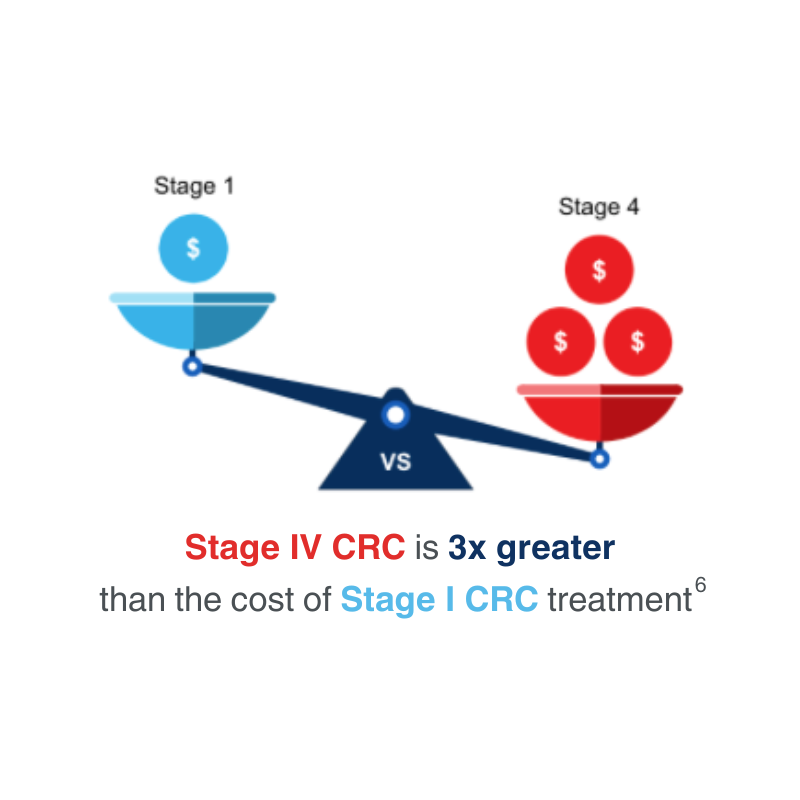
Early-stage diagnosis leads to lowered treatment costs
Benefits of blood-based CRC screening

special
preparation

dietary
changes

sedation

away from
family or work
Our technology achieves high sensitivity by applying a multimodal approach to detect CRC signals in the bloodstream, including DNA that is shed by tumors^7,8,9
Shield™ assesses for DNA shed by tumors, called circulating tumor DNA (ctDNA), and protein to detect colorectal cancer through a simple blood draw

Genomic alterations
- Assessment of ctDNA mutations known to be present in cancer
Epigenomic Modifications
- Analysis of which genes are turned on or off throughout the genome
- Assessment of ctDNA fragment size can indicate the presence of cancer

Shield™ integrates these signals to produce an actionable result
Results:


ECLIPSE is one of the largest cancer-screening studies of its kind to evaluate the accuracy of our blood test for detection of colorectal cancer (CRC)
-
Validated Shield in more than 20,000 patients.
-
Included a diverse population representative of U.S. demographics with broad ethnic and socioeconomic backgrounds.10
-
Patients aged 45-84 at average risk for CRC.

*Sensitivity: Ability to identify the presence of cancer
**Specificity: Ability to identify the absence of advanced adenomas or cancer 90% Specificity (normal colonoscopy)
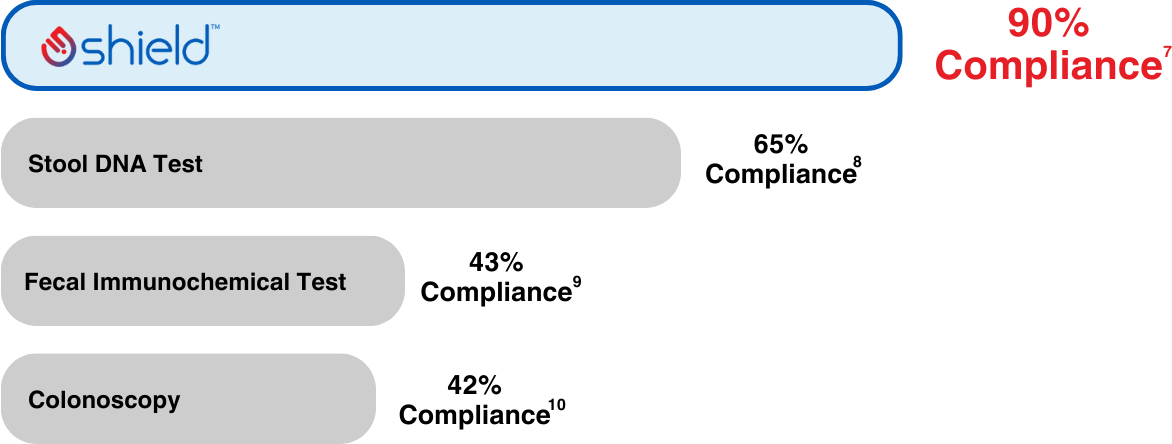
Shield demonstrated 90% compliance in real-world clinical setting11
With more than 20,000 tests completed since Shield launched in 2022, more and more individuals are beginning to see the benefits.
CRC screening strategies combining clinically meaningful performance with high adherence "effective sensitivity," such as a blood-based test, yields more favorable health outcomes (demonstrated by the CAN-SCREEN model).^12
We partner with leading employers like you to help increase CRC screening compliance.
Shield can be easily integrated or adopted to your current on-site cancer screening and prevention program.
Whether it's through your worksite health center or through an onsite screening program, Guardant Health will partner with you to help screen your employees.
Setting you up for success
From educational materials to blood collection kits, we will provide you with the tools and resources you need.
Guardant Health resources
*Services provided by an independent third-party contracted by Guardant Health
About 
At Guardant Health, we’re dedicated to the belief that precision oncology has the extraordinary power to improve patient outcomes. By looking at the unique dimensions of cancer found in blood, including genomic alterations, methylation, and fragmentomics, we are unlocking insights that can increasingly help patients across all stages of cancer – including at its earliest, when it’s most treatable.
By partnering with Guardant Health, you can help your employees access the latest advancements that can ensure the best outcomes for them—and potentially help reduce cancer treatment costs for your organization.

Screen more employees. Achieve more compliance.
Learn more about how you can bring Shield to your company and help increase CRC screening compliance.
- The assay is intended to be complementary to and not a replacement for current recommended colorectal cancer screening methods
- Patients with an “abnormal signal detected” Shield result should be referred for colonoscopic evaluation
- A “normal signal detected” Shield result does not preclude the presence of colorectal neoplasia, and patients should continue participating in guideline-recommended screening programs
- Shield was developed, and its performance characteristics determined, by the Guardant Health Clinical Laboratory in Redwood City, CA, USA, which is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) as qualified to perform high complexity clinical testing. This test has not been cleared or approved by the US FDA
